|
 |
|
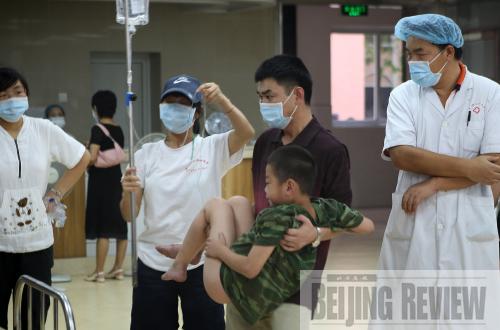
VULNERABLE GROUP: A feverish student receives treatment at Guangdong Province's No. 2 Worker Hospital. On July 13, 32 children attending a summer camp developed fevers. Fourteen were later confirmed to have the A/H1N1 virus (CFP) |
China's health authorities on July 13 altered their directives to diagnose and treat patients infected with the A/H1N1 flu. The change is the second after they altered the plans on May 8.
The revised version states patients with mild symptoms may receive treatment at home but community physicians must monitor for changes at least twice a day. Any differences in condition or temperature are to be reported to health departments.
The modified directive also lists infected persons showing no symptoms as the main source of infection for the first time. Researchers have found that 10 percent of those carrying the virus show no fever but are still infectious.
New measures
Early on July 8, the Ministry of Health (MOH) issued a notice adjusting A/H1N1 flu prevention and control measures, saying that people in close contact with flu patients will no longer be quarantined in reserved places but at home, while patients with mild symptoms may also receive treatment at home.
"The adjustment does not give up supervising close contacts. It's just a different place to quarantine people. They will still be restricted from going outside and making contact with other people," said Liang Wannian, Deputy Director of the MOH Emergency Office.
Those considered to be close contacts were also narrowed in the update. Close contacts include those who diagnosed, treated, nursed or visited a patient, those who worked or lived with a patient and people who had contact with a patient's bodily fluids. On aircraft, close contacts are now considered to be the eight passengers immediately surrounding a patient, the notice stated.
The directive gives provincial health departments the discretion to offer in-home quarantine and treatment.
Medical researchers now believe the virus is no more fatal than the seasonal flu and can be cured, said Liang.
"That doesn't mean we'll let go of the oversight and management of suspected cases as the virus is still spreading across the country," he said. "People will be quarantined in their own homes instead of the current designated places. The new measure will be more cost-effective and sustainable."
The new plan states that patients should live by themselves and use separate washrooms whenever possible when in home quarantine. They must also wear surgical masks when going out in public. Further, they must be quarantined at home for at least seven days. If they must leave home, such as to travel to a hospital, they must wear surgical masks.
The plan also asks community physicians to monitor changes in patients and report the results to health departments. In the event that patients get worse, those physicians must transfer the sick to designated hospitals as soon as possible.
The revised version also lists groups of patients who should not be given the option to receive treatment at home, including children under five years old; the elderly; pregnant women; people suffering from respiratory, cardiovascular (excluding hypertension), kidney, liver, blood, nervous system and neuromuscular, metabolic and endocrine system diseases; those with immune function suppression; and those living in nursing homes or other healthcare facilities.
The MOH said 49 new cases of the
A/H1N1 flu were confirmed on the Chinese mainland from 6 p.m. on July 13 to 8:30 a.m. on July 15, putting the total number of cases at 1,403. No fatal or critical cases of the flu have been reported on the Chinese mainland.
"Although the A/H1N1 flu is still going on, China is well prepared to contain an outbreak," Health Minister Chen Zhu said.
Working on a vaccine
China announced that it would stockpile enough of the A/H1N1 flu vaccine for 1 percent of the population by October 1.
The stockpile of 13 million injections will be used as a preventive measure to counter any sudden outbreak, Liang said, noting the vaccine would be targeted at high-risk groups—those with illnesses that make them more vulnerable and pregnant women.
The first batch of China's A/H1N1 flu vaccine, developed by 10 companies, is expected to be ready by the end of July. The medicine will undergo a two-month clinical trial before it is made available to the public, he said.
One of the 10 vaccine makers, Beijing Sinovac Biotech Co. Ltd. announced recently that it had completed construction of the
A/H1N1 virus seed bank that is necessary to produce a virus antigen.
The company received a seed virus sample from the U.S. Centers for Disease Control and Prevention on June 8 and started producing the vaccine the same day.
Sinovac said it expected to complete production of the first batch and initiate clinical trials of the vaccine by the end of July. The flu vaccine has been named PANFLU.
The company received 100 million yuan ($14.6 million) on June 29 as a loan from the Bank of Beijing. The money will be used to purchase raw pharmaceutical materials and to expand its manufacturing facility, said Yin Weidong, President of Sinovac.
Shenzhen-listed Hualan Biological Engi-neering Inc., another vaccine producer, said on July 1 that its equity-holding subsidiary Hualan Biological Vaccine Co. Ltd. had borrowed 20 million yuan ($2.9 million) from each of two shareholders to fund its vaccine development effort. Hualan can now manufacture nearly 200 million doses annually.
Zhao Kai, a medical virologist and academician at the Chinese Academy of Sciences, said Chinese A/H1N1 flu vaccine production is only for testing purposes and for use in research labs, not for the general public. He said the vaccine would be manufactured and available for use after the relevant departments verify its safety and effectiveness.
The vaccine is intended to deal with a possible seasonal flu outbreak in the autumn and winter. | 