| China |
| Rays of Hope | |
| Trial of treatments for COVID-19 gathers steam | |
|
|
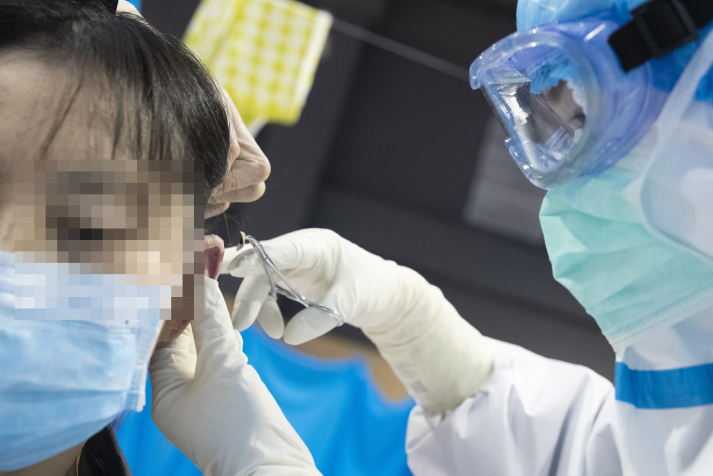 Tu Li, head nurse of the First Hospital of Hunan University of Chinese Medicine, uses auricular therapy on a patient in the Jiangxia Hospital in Wuhan, Hubei Province, on February 25 (XINHUA)
At the Jiangxia Hospital, a hospital converted from the Dahuashan Outdoor Sports Center in Jiangxia District in Wuhan, a national team of over 200 traditional Chinese medicine (TCM) experts are using TCM to treat mildly infected patients. Among the converted hospitals in Wuhan, the city where the novel coronavirus disease (COVID-19) epidemic has been the most severe, the Jiangxia Hospital is the only one to provide mainly TCM treatments.
Every morning, TCM experts, after examining patients' pulse and tongue, give them decoctions to treat COVID-19. Patients are also given granulated medicine for fever, cough, anxiety and insomnia. Auxiliary treatments are used as well, such as auricular therapy, stimulating of pressure points on the ear, and moxibustion, burning moxa, a cone or stick made of ground mugwort leaves, on or near the body's meridians and pressure points. In addition, patients are taught tai chi, a Chinese martial art practiced for both defense and health benefits. For patients who need them, the hospital also provides some Western treatments. According to the National Administration of Traditional Chinese Medicine (NATCM), by February 21, nearly 3,200 TCM doctors from across the country had been sent to Hubei Province in central China where Wuhan is located. Of them, 700 took up their posts at four hospitals in Wuhan, including the Jiangxia Hospital.
Doctors of Peking University Third Hospital, a renowned hospital in Beijing, say goodbye to a patient to be discharged from a ward in Wuhan on February 19 (COURTESY PHOTO)
Combining East and West
TCM prescriptions are part of the national treatment guidelines for COVID-19. According to the NATCM, by February 17, over 60,000 patients confirmed with COVID-19, or 85.2 percent of all confirmed cases, had been treated with TCM. The NATCM has conducted clinical trials in Shanxi, Hebei, Heilongjiang and Shaanxi provinces to select effective prescriptions and promote them nationwide. "We have introduced TCM since the onset of the epidemic and combined it with Western medicine in the treatment. Clinical practice indicates that combined Chinese and Western medical treatment is effective in treating the novel coronavirus pneumonia," Yu Yanhong, an NATCM official, said at a press conference held by the State Council Information Office in Wuhan on February 20. "In mild cases, TCM can help treat fever, cough and lack of strength, reduce the time of hospitalization, and prevent mild cases from developing into serious ones. In critical cases, TCM can alleviate symptoms and reduce the death rate," Yu added. According to her, research has shown that mildly infected patients who received a combination of Chinese and Western medical treatment had their clinical symptoms disappear two days earlier than the normal time. Their temperature returned to normal 1.7 days earlier and hospitalization time was reduced by 2.2 days on average. TCM has also been administered to those who have been in close contact with the infected or those susceptible to the disease to improve their immunity and prevent and reduce infection. It is also used on patients with mild symptoms. 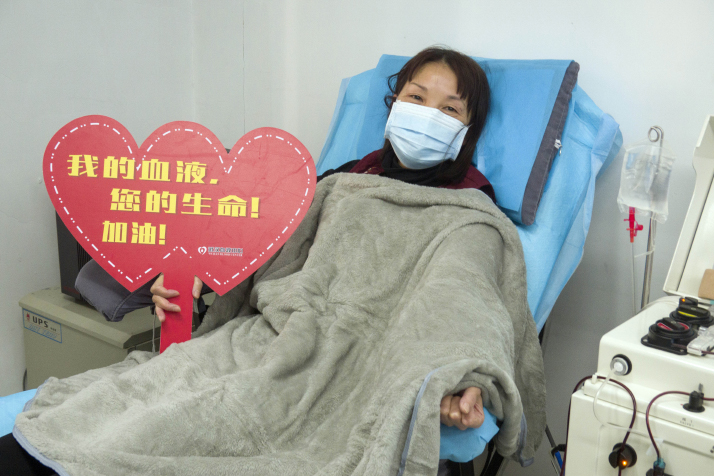 Cai Taoying, a nurse who has recovered from COVID-19, donates plasma in Wuhan on February 17 (XINHUA)
Multiple measures Blood plasma collected from people who have recovered from COVID-19 is being used in critical cases. On February 14, research proving the effectiveness and safety of using plasma from recovered COVID-19 patients to treat critically ill patients passed ethical review at the Jinyintan Hospital in Wuhan. The treatment has also been included in the national treatment guideline. Zeng Fandian, a professor of clinical pharmacology at Tongji Medical College in Wuhan, explained the use of plasma in the treatment of COVID-19 patients in an interview with Wuhan Evening News. When the virus attacks, the human body produces antibody to fight the invader. After the patients recover, the antibody remains in their plasma. Zeng compared the treatment to patients borrowing "weapons" from recovered patients to combat the virus. He said the method has been used in many epidemic outbreaks before, including the severe acute respiratory syndrome and Ebola. "As COVID-19 is a new infectious disease and has no effective medicine, the plasma treatment is used based on past experiences. However, it's an explorative treatment and its effectiveness and safety have yet to be proven through clinical trials," he added. Liu Bende, Vice President of the Jiangxia First People's Hospital in Wuhan, told Wuhan Evening News that plasma treatment can greatly reduce the death rate for critically ill patients but it cannot be applied to all patients. The elderly, those who have other serious diseases and those allergic to plasma cannot be treated with it. Also, patients who have had respiratory or cardiac failure or other organ failure should avoid the treatment in case the plasma transfusion puts greater pressure on their heart and exacerbates damage to other organs. The donors also have to meet multiple requirements. For instance, they should be confirmed COVID-19 patients who were hospitalized for treatment and discharged at least one week before the plasma donation. They should also be between 18 and 55 years. Female donors should weigh at least 45 kg and males above 50 kg. They should have no chronic diseases such as diabetes or cardiovascular and cerebrovascular diseases. Not all cured patients are qualified or willing to donate their plasma, leading to a shortage of plasma. Deng Yiyu, head of the Department of Critical Care Medicine at the Guangdong Provincial People's Hospital in south China, told 21st Century Business Herald that the plasma treatment should be used only for select critically ill patients. The amount of plasma required is large. A critically ill patient might need the plasma of seven to 10 recovered patients with the same blood type. Quan Jun, a professor with the Infectious Disease Department of the Xiangya Hospital, told the National Business Daily that even if a plasma transfusion helps clear the virus, the inflammation it has already caused might still harm the organs. Therefore the treatment may not be effective for all patients. Chloroquine phosphate, an anti-malarial drug, has also been confirmed to have some curative effect on COVID-19. The drug, in use for more than 70 years, was selected from tens of thousands of existing drugs after multiple rounds of screening. It was under clinical trial at over 10 hospitals in Beijing, as well as in Guangdong and Hunan Province in central China. Finally, it was also included in the national treatment guideline for COVID-19 published on February 19. Potential treatment methods Xu Nanping, Vice Minister of Science and Technology, said at a press conference on February 21 that three treatment methods are undergoing large-scale trial. The first is Favipiravir, an antiviral drug that has shown potential in treating the novel coronavirus. Trials conducted in Shenzhen in Guangdong have shown promising results. However, experts suggest the trials be expanded to test patients' tolerance. The second is mesenchymal stem cell treatment. Mesenchymal stem cells have a high self-renewal rate and are distributed widely in the body. This treatment was used on four critical patients who recovered but its clinical trial needs be expanded. The third is the United States biotech company Gilead Sciences' experimental drug Remdesivir. At present 10 hospitals are participating in the clinical trial of the drug, involving over 200 critically ill patients and more than 30 mild cases. Chinese scientists are also racing to develop vaccines. Zeng Yixin, Deputy Director of the National Health Commission, said at a news conference on February 21 that some projects have entered the stage of animal testing. "[We] foresee that as soon as April to May, some vaccines could enter clinical trial, or under specific conditions, could be applied for emergency use," he said. "Our goal is that if required by the outbreak situation, the emergency use of vaccines, as well as the emergency review and approval process, can be activated in accordance with the law." Copyedited by Sudeshna Sarkar Comments to jijing@bjreview.com |
|
||||||||||||||||||||||||||||
|